- Home
- About Us
- Advisory Board
- Products
- Services
- News
- Distributors
- Contact Us
- Search
- Lot I-10-7, D7 Street, Saigon Hi-Tech Park, Tang Nhon Phu Ward, Thu Duc City, HCMC(+84) 28 7301 3688Info@wembleymed.com.vn
We take pride in providing advanced sterilization services and supporting customers in the healthcare and medical device manufacturing sectors.
At WEMBLEY MEDICAL, our strength lies in the combination of cutting-edge technology and high-quality standards. The sterilization facility at WEMBLEY MEDICAL is equipped with modern machinery, utilizing Ethylene Oxide (ETO) sterilization methods, in full compliance with ISO 13485:2016 and following the guidelines of ISO 11135. This ensures that all our customers' products are sterilized efficiently, safely, and cost-effectively.


High Quality: We are committed to providing top-tier sterilization services, ensuring that the quality of medical products is optimally preserved.
Modern Technology: Our advanced equipment utilizes Ethylene Oxide sterilization to ensure consistent performance and quality during the sterilization process.
ISO Certified: Our sterilization procedures fully comply with international standards, following strict guidelines.
Comprehensive Service: WEMBLEY MEDICAL not only provides sterilization services but also offers consultation and support to help clients better understand the sterilization process, ensuring they can effectively evaluate the results after sterilization.
Choose WEMBLEY MEDICAL to ensure that your products are well-protected and meet all necessary quality standards.
Learn more about our Ethylene Oxide (ETO) Sterilization service:
Ethylene Oxide (also known as EO or ETO) is widely used to sterilize medical devices and healthcare instruments. The process involves exposing products to Ethylene Oxide gas in a controlled environment. Because it's a low-temperature method, ETO sterilization ensures product safety without affecting the materials.
Ethylene Oxide sterilization is a chemical process that includes four key elements: gas concentration, humidity, temperature, and time. ETO is an alkylating agent that disrupts microbial DNA, preventing it from reproducing. ETO penetrates through breathable packaging, sterilizing all accessible surfaces of the product by alkylating the proteins necessary for cellular reproduction.
Ethylene Oxide is a gas that can penetrate multiple layers of breathable packaging, making it suitable for sterilizing many types of materials that are incompatible with other sterilization methods, including:
Products with complex assembly parts.
Catheters and tubular products.
Products that cannot withstand or are limited to high temperatures.
Devices with electronic components.
Surgical instruments and medical devices that require sterilization.
Gauzes, tapes, and medical clothing.
When choosing a sterilization service provider, manufacturers should select a contractor with technical expertise, flexibility, and the necessary certifications to ensure product reliability, as well as guidance for future certification registration of these products.
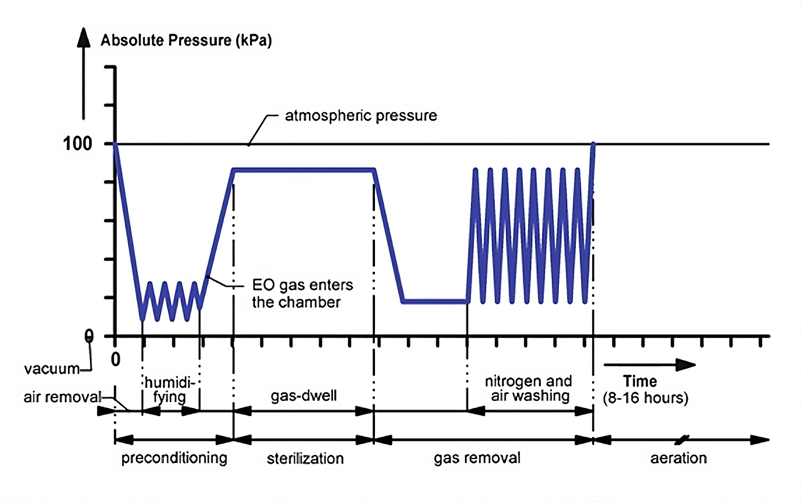
Pre-conditioning: This involves humidifying and heating the product under predetermined conditions. This ensures optimal ETO sterilization performance, even in the most challenging areas of the product.
ETO Exposure: This stage involves processes that meet the required ETO exposure levels to ensure effective product sterilization.
Aeration: This step accelerates the removal of residual ETO gas from the sterilized products, ensuring that the residual levels comply with the limits set by ISO 10993-7.